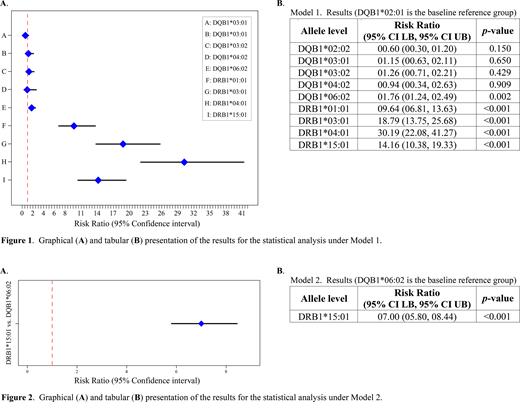
The HLA haplotype DRB1*15:01/DQB1*06:02 has been shown to be associated with the development of inhibitory antibodies ("inhibitors") against therapeutic FVIII (tFVIII) proteins in Hemophilia A (HA) patients. Moreover, this same haplotype has also been implicated with an increased risk for the development of multiple sclerosis, narcolepsy, and drug-induced-hypersensitivity and -liver-injury. Recent reports in the literature have indicated that the alleles of this haplotype may have differential effects on immunogenicity despite the fact that they are in strong linkage disequilibrium (LD). Thus, the question arises as to which of the two constituent alleles of this haplotype is the true HLA risk allele. To address this question we analyzed peptidomic profiling data on HLA-class-II (HLAcII)-presented peptides from dendritic cell (DC)-protein processing and presentation assays (PPPAs) performed in two independent experiments performed by Peyron et al. (2018) and Diego et al. (2020) which reported results on both the HLA-DQ and -DR isomers. The tFVIII in both experiments is full-length recombinant FVIII (FL-rFVIII). We performed log-linear mixed model analyses under two models, where the dependent variable in both models is the logarithm of the expected peptide count. Under Model 1, we analyzed in the fixed effect component of the model a single HLA allele predictor variable consisting of 10 levels represented by distinct DRB1 and DQB1 alleles (4 and 6 levels, respectively). Under this model, we analyzed in the random effects component of the model variables for individuals (8 and 9 levels from Peyron et al. and Diego et al., respectively), experiments (2 levels for Peyron et al. and Diego et al.), and HLA isomers (2 levels for DQ and DR). The random effects component of the model serves to reduce the error (or "noise") in our estimation of the predictive effect of HLA alleles for the peptide counts by accounting for the clustering due to individuals, experiments, and isomers. This approach therefore can be understood as maximizing the signal-to-noise ratio. Model 2 was more focused in that the single HLA allele predictor variable in the fixed effect component consisted of only DQB1*06:02 and DRB1*15:01. Model 2 was also simpler in that we only accounted for two random effect variables, namely for individuals (in this case 2 and 6 levels for Peyron et al. and Diego et al., respectively) and experiments. Thus, Model 2 is a direct head-to-head comparison of the two main HLA alleles of interest. The Model 1 results are reported in Figure 1A and B, where it can be seen that relative to the baseline reference allele provided by DQB1*02:01, both DQB1*06:02 and DRB1*15:01 are at significantly increased risk of contributing to the overall peptide count. Results are reported as risk ratios (RRs) and their associated 95% confidence interval lower and upper bounds (95% CI LB and 95% CI UB). For DQB1*06:02 and DRB1*15:01 respectively, we found a RR (95% CI LB, 95% CI UB) of 1.76 (1.24, 2.50) and 14.16 (10.38, 19.33). Because they are compared to the same baseline, the two RRs may also be directly compared, thus showing that DRB1*15:01 contributes significantly more to the overall peptide count than DQB1*06:02. Under Model 2 (the head-to-head comparison), the RR for the DRB1*15:01 allele against the baseline DQB1*06:02 allele is 7.00 (5.80, 8.44) (Figure 2A and B). These results contribute to the field of precision medicine because they demonstrate that even in the case of alleles in tight LD, their effects can be effectively disentangled by a DC-PPPA and a log-linear mixed model analysis. Our results support the conclusion that in regard to the well-known risk haplotype of DQB1*06:02/DRB1*15:01, the true HLA risk allele seems to be DRB1*15:01.
Luu:Haplogenics Corporation: Current Employment. Hofmann:CSL Behring: Current Employment. Powell:Haplogenics Corporation: Membership on an entity's Board of Directors or advisory committees. Dinh:Haplogenics Corporation: Current Employment. Escobar:National Hemophilia Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Maraskovsky:CSL Behring: Current Employment. Howard:Haplogenics Corporation: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal